Kossel-Lewis Approach to Chemical Bonding
Kossel-Lewis Approach to Chemical Bonding: Overview
In this topic, we will understand the formation of chemical bonds in terms of electrons. It introduces us to the different terms related to the chemical bonds and facts given by Kossel and Lewis.
Important Questions on Kossel-Lewis Approach to Chemical Bonding
Mention the fluoride compounds of the noble gases.
What are the formal charges on and in the Lewis (electron dot) structure of the phosphate oxyanion represented in the figure?
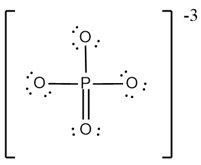
Give 2 drawbacks of Kossel's Postulate.
Mention few applications of formal charges?
_____ is a factor based on a pure covalent view of bonding in which electron pairs are shared equally by neighbouring atoms.
Formal charges do not indicate real charge separation within the molecule.
How will you calculate the valency of potassium?
Define the group valency.
List some odd electron molecules.
Identify odd-electron molecules from the following:
Which of the following oxides is an odd-electron molecule.
Which of the following bonds can be present in a chemical compound?
Hydrogen gas is a chemical compound.
Which of the following is a Chemical Compound?
Which of the following is not a Chemical Compound?
In molecule, the number of bond pairs and lone pairs of electrons are .
In the Lewis structure, the formal charge on the central atom of is
Aluminium chloride is a Lewis acid because it can donate electrons.
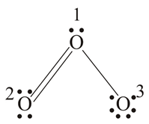
In ion formal charge on the oxygen atom of bond is
